Fuel Cell
- José M Soria
- 6 abr 2023
- 4 Min. de lectura
Actualizado: 22 abr 2023
What is a fuel Cell?
A fuel cell is a "Factory" that takes fuel as input and produces electricity as output. Like a factory a fuel cell will continue to churn out product (electricity) as long as raw material (fuel) is supplied. This is the key difference between a fuel cell an a battery. FC is really a factory, a shell, which transforms the chemical energy stored in a fuel into electrical energy.

But viewed this way , combustion enginers are also "chemical factories". Combustion engines also take the chimical energy stored in a fuel and transform it into useful mechanical or electrical energy.
Combustion engine
So what is the difference between a combustion engine and a fuel cell? In a convention combustion engine, fuel is burned, releasing heat. Consider the simplest example, the combustion of hydrogen:
H2 +1/2O2 = H2O
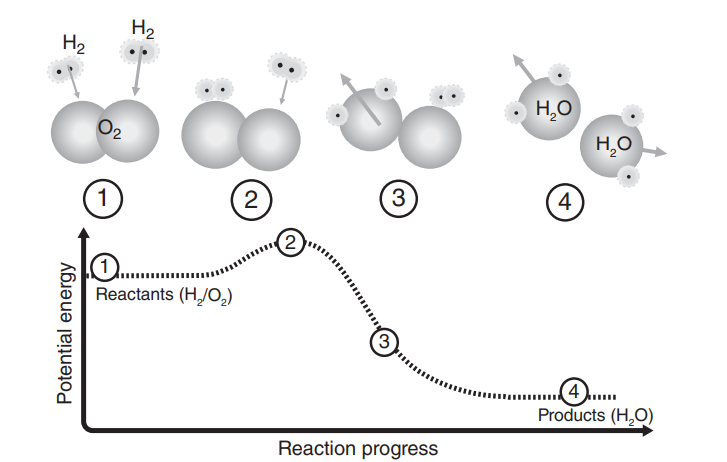
On the molecular scale, collisions between hydrogen molecules and oxygen molecules result in a reacction. Speciafically, at the atomic scale, in a matter fo picoseconds, (1) hydrogen-hydrogen bonds an oxygen-oxygen bonds are broken, must first be broken requiring energy input(2) before hydrogen-oxygen bonds are formed, lending to energy output (3,4). The bonds are broken and formed by the transfer of electrons between the molecules. The energy fo the products water bonding configuration es lower than teh bonding configuration of the initial hydrogen and oxygen gases. This energy difference is released as heat. Although the energy difference between the initial and final states occurs by a reconfiguration of electrons as they move from one bonding state to another. This energy is recoverable only as heat because the bonding reconfiguration occurs in piscoseconds at an intimate, subatomic scale. To produce electricity, this heat energy must be converted into mechanical energy, and the mecanical energy must be converted into electrical energy.
Bonds and Energy

The atoms always prefer to be together instead of alone. When atoms como together, they form bonds, lowering their total energy. When the hydrogen atoms are far apart from one another (1), no bond exists and the system has high energy. As the hydrogen atoms approach one another, the system energy is lowered until the most stable bonding configuration (2) is reached. Further overlap between the atoms is energetically unfavorable because the repulsive forces between the nuclei begin to dominate (3)
A simple fuel cell
Consider an alternative solution: to produce electricity directly form the chemical reaction by somehow harnessing the electrons as they move from high-energy reactant bonds to low-energy product bonds. In fact, this is exactly what a fuel cell does. But how do we harness electrons that reconfigure in picoseconds ata subatomic lenghth scales?
The answer is to spatilally separate the hidrogen an oxygen reactants so that the electron transfer necesary to complete teh bonding reconfiguration ocurs over a greatly extended length scale. Then, as the electrons move from the fuel species to the oxidant species, they can be harnessed as an electrical current.
Basic fuel cell opartion
ANODE=OXIDATION; CATHODE=REDUCTION
To understand any discussion ofelectrochimistry, it is essential to have a clear concept of the terms oxidation, reduction, anode, and cathode.
Oxidation and Reduction
Oxidation refers to a process in which electrons are removed from a species. Eletrons are liberated by the reaction.
Redution refers to a process in which electrons are added to a species. Eletrons are consumed by the reaction.
In a electrochemical half reactions that occur in a H2-O2, fuel cell
H2 = 2H+ + 2e-
1/2O2 + 2H+ 2e- = H20
The hydrogen reaction is an oxidation because electrons are being liberated by the reaction. The Oxigen reaction is a reduction reaction because electrons are being consumed by the reaction. The preceding electrochemical half reactions are therefore known as the Hydrogen oxidation reaction (HOR) and the Oxygen reduction reaction (ORR)
Anode and cathode
Anode refers to an electrode where oxidation is taking place. More generally, the anode of any two-port device, such as a diode or resisto, is the electrode where electrons flow out.
Cathode refers to an electrode where reduction is taking place. More generally, the cathode is the electrode where electrons flow in.
For a hydrogen-oxygen fuel cell:
The anode is the electrode where the HOR takes place
The cathode is teh electrode where the ORR takes place.
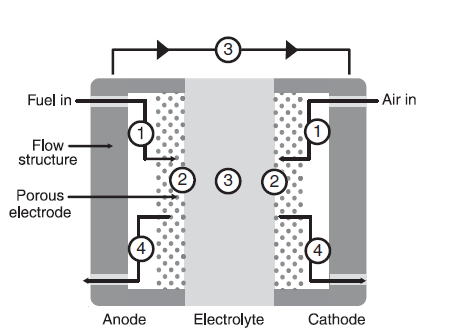
The figure above shows a detailed, cross-secctional view of a planar fuel cell. A quick tour, to understand the physics behind, sequentially, as numbered on the drawing, these steps are as follows:
Step 1: Reactant Transport. When a fuel cell is operated at high current, its demand for reactants is voracious. If the reactants are not supplied to the fuel cell quickly enough, the device will "starve". Efficient delivery of reactants is most effectively accomplished by using flow field plates in combination with porous electrode structures. The shape, size, and pattern of flow channels can significantly affect the performace of the fuel cell. Understandig how flow structures and porous electrode geometries influence fuel cell performace is an exercise in mass transport, diffusion, anand fluid mechanics.
Step 2: Electrochemical Reaction.
Step 3: Ionic (and Electronic) Conduction
Step 4: Product Removal
Fuel Cel Performace
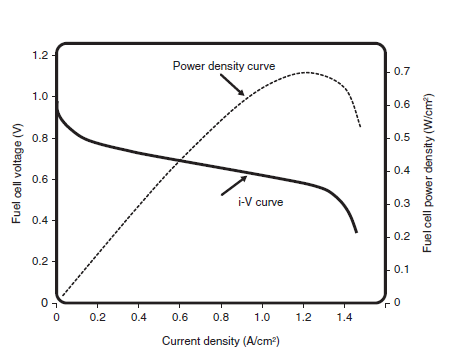
V = Ethermo − 𝜂act − 𝜂ohmic − 𝜂conc


Comentarios